Summary:
In children with mild-moderate persistent asthma, as defined by symptoms and positive methacholine challenge, treatments for 12 months with (1) an inhaled corticosteroid (ICS), (2) an ICS @ 50% dose combined with a long-acting beta-agonist (LABA), and (3) an oral leukotriene receptor antagonist (LTRA), do not differ in their effects on asthma control, as measured by the percentage of days without asthma.
Design:
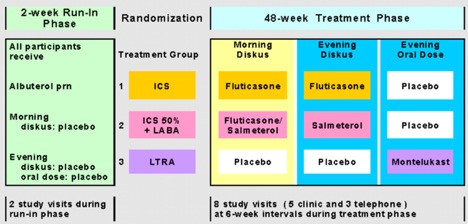
After a 2-4 week assessment/characterization run-in period, 6-14 year-old children who met NAEPP criteria for mild-moderate persistent asthma specifically based on symptom criteria and methacholine PC20 ≤ 12.5 mg/ml and FEV1 ≥ 80% were randomized to one of the three active treatment arms for 12 months. Randomization was stratified according to clinical center, bronchodilator response (< 12% or ≥ 12%), race (Caucasian or nonCaucasian), and methacholine PC20 (< 2 or ≥ 2 mg/ml). The primary outcome variable was the proportion of asthma-free days during the 12-month treatment period. Secondary outcomes included other measures of asthma control (percentage of rescue-free days, albuterol-free days, and episode-free days; the number of asthma exacerbations requiring prednisone therapy and the time to the first asthma exacerbation), forced oscillation and spirometry, reversibility (FEV1 pre- and post 2 puffs of albuterol MDI), methacholine PC20, exhaled nitric oxide, and asthma-related quality of life.
IMAGE TO COME
Population
Childhood Asthma Research & Education (CARE) Network
Enrollment for PACT began in October 2002 and the last patient visits occurred in January 2005 with 285 randomized children. Fluticasone monotherapy and PACT combination were comparable in many patient measured outcomes, including percent of asthma control days, but fluticasone monotherapy was superior for clinic-measured FEV1/FVC (p=0.015), maximum bronchodilator response (p=0.009), eNO (p<0.001), and PC20 (p<0.001). Fluticasone monotherapy was superior to montelukast for asthma control days (64.2% versus 52.5%, p=0.004), and for all other control outcomes. Growth over 48 weeks was not statistically different (fluticasone 5.3 cm, PACT combination 5.3 cm, montelukast 5.7 cm). The major publication appeared in JACI:
Sorkness CA, Lemanske RF Jr, Mauger DT, Boehmer SJ, Chinchilli VM, Martinez FD, Strunk RC, Szefler SJ, Zeiger RS, Bacharier LB, Bloomberg GR, Covar RA, Guilbert TW, Heldt G, Larsen G, Mellon MH, Morgan WJ, Moss MH, Spahn JD, Taussig LM. Long-term comparison of three controller regimens for mild-moderate persistent childhood asthma: The Pediatric Asthma Controller Trial (PACT). J Allergy Clin Immunol 2007; 119: 64-72. PMID: 17140647

